Equipment:
- Wide mouth glass trough [glassware cabinet]
- Water boiler [B3]
- Thermochromic painted metals apparatus [D3]
Demo:
- Fill the water boiler with enough water to fill the trough up to the exposed metal of the thermochromic painted metals apparatus.
- Once the water is boiled, carefully pour it into the trough so that the exposed metals are partially submerged in the hot water.
- Watch the thermochromic paint activate throughout the metals.
Explanation:
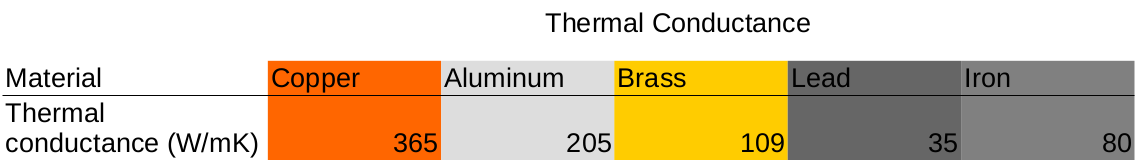
This demonstration shows the heat conductivity of various metals, painted with thermochromic paint, by exposing them to boiling water. As the paint reaches 95°F/35°C, it changes from black to yellow. The thermochromic paint allows one sees how well the metals conduct heat by the “speed” that the paint changes color.
Thermal conductance is the ability for a material to transmit heat through itself. The general equation for thermal conductivity is shown below
Where q is the heat flux in the rate per unit area of heat flow, k is the thermal conductivity of the material, T1 is the temperature at one point in the material, T2 is the temperature at another, and L is the distance between the two points.
Thermal conductivity is generally dependent on the density of the material. More dense materials typically have higher thermal conductivity. As temperature affects the density of metals, an increase in temperature causes a decrease in thermal conductivity.
Written by: Finn Amend