
Figure 1
Materials:
- Large bell jar
- Aluminum base and rubber pad
- Watch glass or glass dish
- Three-neck flask filled with drying pellets
- Vacuum pump and hose setup
- Cold water and ice to cool it
- Stand made of thermally insulating material (we use cork)
Demo:
- Cool water with ice and fill a watch glass halfway with the ice-cold water
- Open the vacuum jar (two people recommended): lift the bell jar and peel away the mat underneath to open easily. Place the watch glass filled with water inside of the vacuum jar on a stand made of thermally insulating material.
- Turn on the vacuum pump; water will begin boiling at about 28mm Hg. It will take about 5-10 minutes for ice will form on the surface, and water will continue to boil below it.
Explanation:
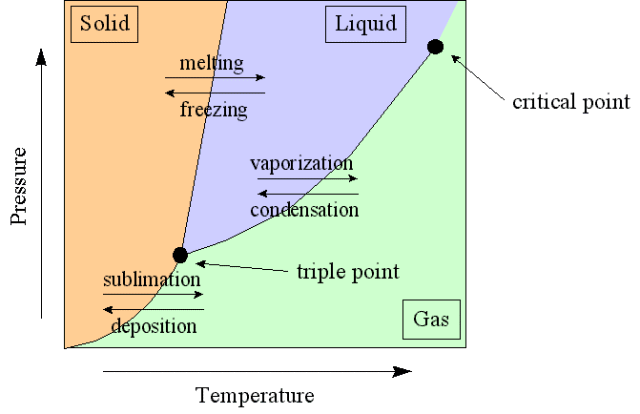
Figure 2 – Phase diagram
In one’s everyday life they experience three phases of materials: solids, liquids, and gases. Water provides some of the most familiar examples of phase transitions. One often observes the freezing or melting of ice, the boiling of water, and the condensation of steam. These are often attributed to changes in temperature, however pressure also plays an important role.
Phases are only stable in certain ranges of pressure and temperature. Transition phases normally occur under conditions of phase equilibrium between two phases, which are indicated by the curves on the phase diagram above (figure 2). For instance, near the bottom of the phase diagram there is a curve indicating the phase transition between a solid and a gas. At this transition, gas sublimates from the material’s solid state, skipping the liquid transition entirely, as seen in dry ice at normal atmospheric pressure.
The triple point occurs where the solid, liquid, and gas transition curves meet. The triple point is the only condition in which all three phases can coexist, and is unique for every material. Water reaches its triple point at just above freezing (0.1° C) and at a pressure of 0.006 atm.
Notes:
Currently only transportable to Thimann Lecture halls.
This demonstration requires at least 20 minutes of lecture time. A video camera is recommended for large lecture classes.
Water evaporates very fast during evacuation, which can be harmful for the vacuum pump. To keep the pump safe, a water vapor trap is installed between the jar and the pump (figure 1). This trap is a three-neck flask filled with blue Drierite crystals (Anhydrous Calcium Sulfate). When the Drierite crystals change color to pink, they do not absorb water any more and must be replaced.