To determine the surface tension of a liquid, a metal ring known as a Du Noüy ring is suspended from a dynamometer: a soft spring inside of a plastic tube. The metal ring is completely submersed in a liquid-filled container, which is then slowly lowered by a jack. As the glass of liquid is lowered away from the ring, the surface tension of the liquid holds on, pulling the spring down. Once the threshold force is reached (measured by the dynamometer), the ring will break free.
Explanation:
Surface tension is the result of an imbalance in cohesive forces between like molecules in a liquid.
Molecules within the bulk of the fluid are attracted through cohesion to neighboring molecules in all directions and experience an approximate net force of zero, while molecules on the surface only experience attractive forces on their lower half, leading to a net force directly into the fluid. It is this imbalance in forces experienced by molecules on the surface of a liquid that leads to a “film” of molecules and ultimately a surface tension.
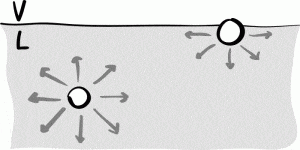
Fig 2. Molecules on the surface experience a net cohesive force into the liquid, while molecules inside the liquid experience cohesive forces in all directions. Image from, American Journal of Physics.
From this, the following equation is used to obtain a value for the surface tension through a relation between the Du Noüy ring’s geometry and the maximum force experienced while pulling the ring out of the liquid at a constant speed:
where
= force (
)
= weight of the ring minus buoyant force on the ring when fully submersed in fluid (
)
= average radius of ring plus radius of wire (
)
= surface tension (
for water,
for ethanol)
The cohesive forces responsible for surface tension are dependent on the shape and structure of the molecules in a liquid, meaning unique liquids can have unique surface tensions. For example, the cohesive forces between water (H2O) comes from hydrogen bonding between the electronegative oxygen atoms attraction to the slightly less electronegative hydrogen atoms in other water molecules (Fig 3).
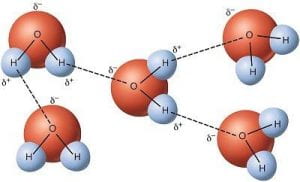
Fig 3. Hydrogen bonding between water molecules. Image from, USGS.
This relation between the polarity of a molecule and surface tension means that liquids of more polar molecules can have larger surface tensions. This can be seen in ethanol (C2H5OH), which has a weaker surface tension than water because the C2H5 group in ethanol is non-polar and the polarity of the OH group is weaker than the polarity of water.
Notes:
- This experiment can be done quantitatively (reading the force gauge) as well as qualitatively (feeling the stronger force required to remove the ring from fluids with larger surface tensions).
Written by Aleksander Beck


