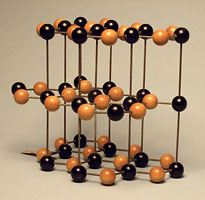
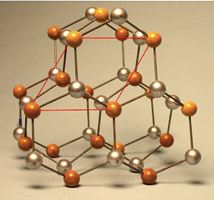
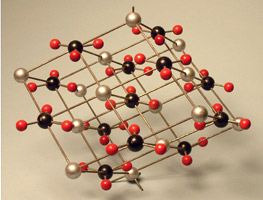
Many crystal structures are available for demonstration. The crystal models are found in [Cabinet G].
- Simple Cubic
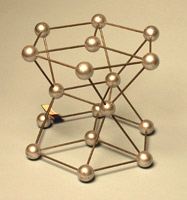
- Body Center Cubic (BCC)
- Face Center Cubic (FCC)
- Diamond Structure
- Magnesium–Hexagonal Close Packed Geometry
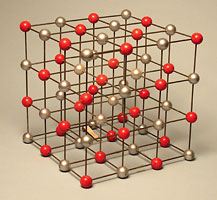
- Sodium Chloride (NaCl)–Cubic Geometry
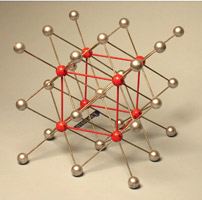
- Cesium Chloride (CsCl)–Cubic and Cubic Interstitial Geometry
- Triclinic Geometry (Example: wollastonite)
- Copper–Face-Centered Cubic Geometry
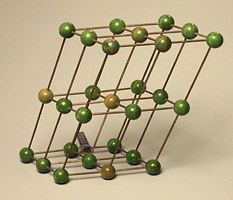
- Hexagonal geometry (Example: graphite)
- Zinc Sulfide–Wurtzite Crystal Geometry
- Calcite (CaCO3)–Trigonal Hexagonal Scalenohedral Geometry
- Zincblende (for example InSb)–Diamond Lattice geometry
Solid state physics started with the discovery of x-ray diffraction of crystals and with calculations that described the electronic nature of crystalline solids. The electronic properties of solids are best expressed via crystals. Atoms or groups of atoms are the building blocks of crystals. Crystal structure is a geometric arrangement of those atoms or groups of atoms in a three-dimensional array of identical building blocks that are bound by a lattice. When studying a crystal structure there are three main parameters; lattice constant, lattice vectors, and the basis.
Crystals in 2D:
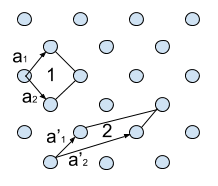
- A lattice is the set of mathematical points that basis is attached to. In 2 dimensions lattice points are described by two basis vectors
and
, with
and
being integers. R is the point from which the vectors start.
- A basis is the group of atoms that can be repeated in infinite repetition to construct an ideal crystal. Once the axes of the crystal have been chosen the basis can be identified. Every basis in the crystal is identical and a crystal can be created by adding a basis to every lattice point. The basis is not unique and just depends on the mathematical constructions of the lattice vectors.
- The lattice constant refers to the physical dimension of the unit cell. Generally, a 3-D lattice will have three lattice constants that are referred to as a, b and c. For cubic lattices, since it is a cube and all of the lengths are the same we only need to define one constant (a).
- A primitive unit cell is a type of unit cell or cell. A primitive unit cell is a minimum volume cell. The most common way of constructing a primitive unit cell is using the Wigner-Seitz method.
Crystals in 3D:
In 3 dimensions, points of a lattice analogously have three integers and vectors:
Simple Cubic Lattice
The most basic 3D lattice is the simple cubic lattice. Each unit cell is a single cube that is repeated. The unit cell has eight corners each with a contribution of 1/8 of an atom. This unit cell adds up to have one atom.
Body-Centered Cubic (BCC) Lattice
BCC lattices consist of a simple cubic lattice, but with an additional atom in the center of the cube. This unit cell contains two atoms. 8×(1/8)=1 from the 8 corner atoms and the addition of one more atom in the center of the unit cell.
A common BCC crystal is that of pure sodium. It forms a BCC lattice with one basis atom: Na at [0,0,0]
Cesium chloride is a similar looking crystal that seems to have a BCC lattice, however it is not BCC. It is simple cubic with a two atom basis. Cs at [0,0,0] and Cl at [1/2,1/2,1/2]. You can think of it as two cubic lattices interlocked.
Face-Centered Cubic Lattice (FCC)
This lattice is a simple cubic lattice with an extra atom at the center of every face of the cube (6 faces total). This conventional unit cell contains four atoms [4=8×(1/8)+6×(1/2)]. Each atom on the face contributes 1/2 of an atom.
Copper is a common crystal that forms a FCC lattice with its basis = Cu at [0,0,0]
Written by: Nick McCabe
Photo Credit: Sophia Sholtz