Part 1: Curie Point for Iron
An iron washer suspended with a stainless steel wire is placed near a strong magnet so that it hangs at an angle (figure 1). When the washer is heated to a glowing red with a propane torch (approximately 770 C), it loses its ferromagnetic properties and swings away from the magnet.
Materials:
- Large horseshoe magnet
- Iron washer attached to a stainless steel wire
- Stand with clamps
- Propane torch
Part 2: Curie Point for Gadolinium
Pure Gadolinium has a Curie Point at 19 C, but our Gadolinium sample has a higher Curie Point due to impurities. A thermocouple probe is inserted into the Gadolinium sample and connected to the large LCD display. The sample is attached to a small powerful rare-earth magnet which is attached to an iron bar (Figure 2). The heat gun is used to warm up the sample until the Gadolinium loses its ferromagnetic properties and falls off at approximately 50 C (Figure 3).
Materials:
- Piece of gadolinium with insert for thermocouple probe
- Thermocouple probe
- Strong rare-earth magnet stack
- Iron rod
- Stand with right angle clamps
- Giant thermometer
- Heat gun
- Bean bag to catch falling gadolinium
Explanation:
The Curie Point of any material is the temperature above which the material will lose its ferromagnetic properties and gain paramagnetic properties. Both materials that we use in this demonstration (iron and gadolinium) are ferromagnetic at room temperature.
1. What is magnetism?
The magnetic force of a material is determined by the magnetic moment of the atoms that compose it. Each electron in an atom has a certain dipole moment, which can be either “up” or “down” (which is affected by the spin of the electron), and the collection of each electron’s dipole moments determines the magnetic properties of a material. Due to the Pauli exclusion principle–whereby an electron can only occupy an orbital with an electron of the opposite spin–when an orbital has a pair of electrons one of them has an “up” spin and the other has a “down” spin. These two spins will cancel each other out so that their combined moment is zero. For this reason, only atoms with unpaired electrons can have a net magnetic moment. The orientation of each atom’s magnetic moment within the material is what determines the material’s certain type of magnetism.
There are several different ways that a material can be magnetic, but the type of magnetism that is the strongest and most commonly associated with the term “magnetic” is ferromagnetism. In addition to being ferromagnetic, a material can be paramagnetic, ferrimagnetic, or antiferromagnetic.
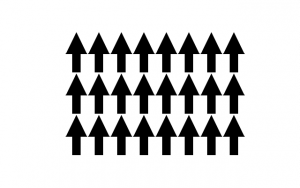
Ferromagnetism is when all the magnetic moments in a material spontaneously order themselves to point in a certain direction. Often, the magnitude of the magnetic moments are equal, but this is not necessary for a material to be considered ferromagnetic. This is the strongest type of magnetism because none of the magnetic dipoles in the material are being cancelled out by any others.
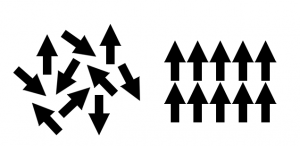
Paramagnetism is when a material’s magnetic moments are disordered unless an external magnetic field is applied. A paramagnet is attracted to a magnetic field because its magnetic moments will align themselves parallel with the magnetic field. A similar, but opposite effect is seen in diamagnetic materials, whose magnetic moments align themselves anti-parallel with the applied magnetic field and are therefore repelled by it. Diamagnetism is usually a much smaller effect than paramagnetism.
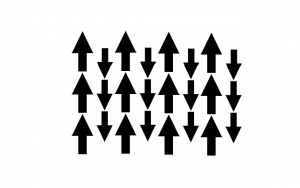
Ferrimagnetism is when a material is composed of several different ions with differing orientations and magnitudes of magnetic moments. Due to the differing orientations and sizes of the magnetic moments, there is a net magnetic moment in the material, but it is smaller than in a ferromagnetic material where all the moments are aligned.
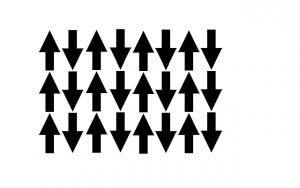
Anti-ferromagnetism is where the moments in a material are of the same magnitude and oriented oppositely, so there is no net magnetic moment in the material.
- Ferromagnetic Moment
- Paramagnetic Moment. Field absent on left, field applied on right
- Ferrimagnetic Moment
- Antiferromagnetic Moment
Source: ACGrain at English Wikipedia [GFDL or CC BY-SA 3.0], via Wikimedia Commons
The “willingness” of a material to be attracted to or repelled by a magnetic field is known as the materials magnetic susceptibility, . A paramagnetic material has a positive magnetic susceptibility and is attracted to a magnetic field, whereas a diamagnetic material has a negative magnetic susceptibility and tends to be repelled by an applied magnetic field.
What is the Curie temperature?
Every material has a unique Curie temperature past which it will exhibit no permanent magnetic (ferromagnetic) properties. A ferromagnet like iron that is heated past its Curie point will stop being strongly attracted to permanent magnets and will instead exhibit smaller, paramagnetic properties.
The Curie law gives the magnetic susceptibility () of a material for a temperature (T) much greater than its Curie temperature:
, where C is the material-specific Curie constant.
The magnetic moments in a material will spontaneously unalign at the Curie temperature due to an increase in thermal noise of the system. As heat is added to a material, the increased internal energy is enough to nudge the magnetic moments out of their parallel alignments. Paramagnetic materials are materials where any amount of thermal noise (any temperature above 0K) can be enough to nudge the system out of alignment.
When a ferromagnetic material is heated past its Curie point it will become paramagnetic, meaning its magnetic moments will align parallel to an applied magnetic field. However, at temperatures very close to the Curie point, the magnetic susceptibility of the material will be very small, and the material will not be noticeably affected by an applied magnetic field (like the permanent magnet present in the demonstration). If the material is heated much past its Curie point, the magnetic susceptibility will increase and it will show a larger paramagnetic effect.
Written by Sophia Sholtz