Demo:
This demonstration consists of two wheels connected by a string of nitinol. Nitinol is a shape memory alloy, which means it changes shape when its temperature increases past a certain threshold temperature. As the smaller wheel is dipped in warm water, the nitinol wire wants to extend to a straight line rather than be curled around a wheel. This causes the wheels to spin, which pulls some of the wire out of the warm water. This cools the wire, returning it to a flexible state so that it once again bends around the wheels. This motion causes the nitinol wire to spin around the two wheels as long as the smaller wheel remains partially submerged.
Materials:
- Water heater and plug (B2)
- Beaker for hot water (Glass Cabinet)
- Thermobile device (D4)
- Camera (large classes)
- Thermometer (water temp must be > 50°C and < 80°C) (D4 [small and large])
Instructions:
- Heat water (~5 min)
- Pour into beaker
- Let water cool to below 80°C
- Lower edge of brass (smaller) wheel into water without submerging bearing
- Wheel should start spinning
Shape Memory Alloys:
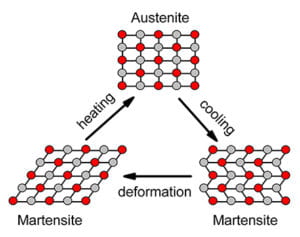
Shape memory alloys (SMAs) are a classification of metal alloys that “remember” their configuration at high temperatures. At cooler temperatures, called the martensite phase, they are malleable and can be formed to different shapes, but when the alloy passes a temperature threshold specific to the SMA alloy, the metal bends back to its original configuration, called the austenite phase. This is possible due to the crystal-like structure of SMAs and their ability to be deformed without changing their molecular structure.
The shape that the SMA “remembers” is set by heating the metal far past the austenite temperature threshold but not so hot that the metal recrystallizes to a non-SMA alloy. This temperature range is different for different SMA alloys but is usually in the range of 400 to 500 degrees Celsius. After the “remembered” shape is set, the metal is quenched in cool water.
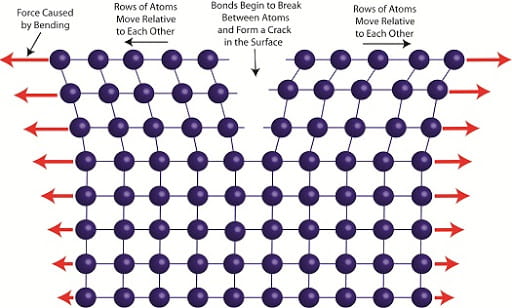
When most metals are bent, their individual atoms break the bonds between one another to conform to the forces that are being applied to the metal as seen in figure 3. SMA’s however, retain the same molecular structure because the atoms shift rather than diffuse. As seen in figure 2, while in the martensite phase, when the metal is deformed, the atoms shift their crystalline structure but keep their bonds to one another. Then when adding heat to go to the austenite phase, these bonds reform to their original shape.
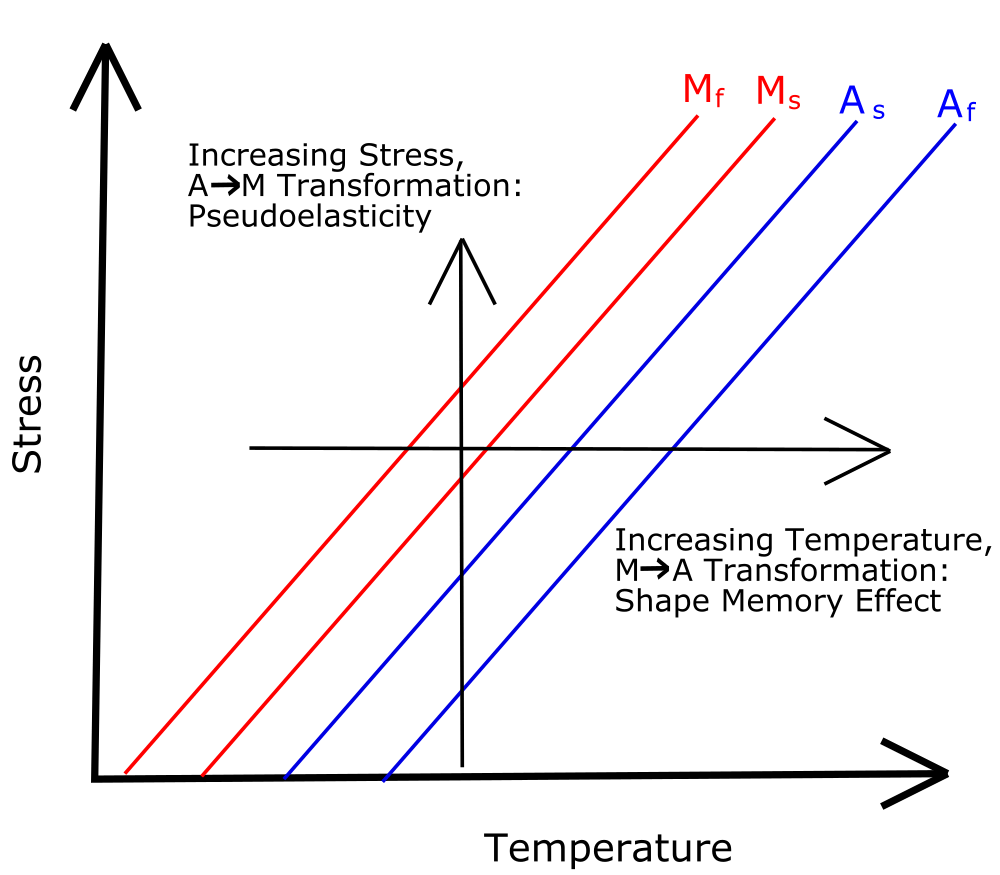
As seen in figure 4, the martensite and austenite phases are not only functions of temperature but stress as well. As the stress increases, the alloy needs a higher temperature to force the atomic structure to the austenite phase. This is because the atomic forces from the temperature must make up for the stress forces pushing the atoms to a deformed, martensite phase.
Superelasticity:
As seen in figure 4, the phases of SMAs not only depend on their temperature, but also on their applied stress. The more stress on the alloy, the higher temperature is needed to revert from martensite to austenite. If the alloy is heated to just above its austenite temperature threshold, it will change to the austenite phase. As seen in figure 4, when stress is applied, a higher temperature is needed for the alloy to be stay in the austenite phase, so the metal will transform back to its martensite phase without a change in temperature. Since the martensite phase is more easily deformed, the alloy will bend quite easily with little stress. As the stress is removed, the temperature stays the same and the alloy transforms back to the austenite phase, causing the alloy to revert to its austenite shape. This effect is called superelasticity.
Uses of SMAs:
SMAs were discovered in 1951 and have shown to be extremely useful for safety and scientific purposes. In many environments where electronics can’t be used due to high temperatures or water exposure, SMAs can be “programmed” to do simple mechanical processes. For example, in some deep fryers, a mechanical lock blocks a user from inserting the fry basket until the oil reaches a desired temperature threshold. Some shower systems implement an SMA spring that introduces cold water when the temperature would cause burning. In the scientific field, SMAs have been used by researchers at the University of Santa Clara to collect data in extreme temperatures such as deep sea vents. By using an SMA to block a spring from activating a syringe, the syringe is only activated at a specific temperature. Once that temperature is reached, the SMA contracts, allowing the syringe to collect a water sample at temperatures that would melt any electronics.
References:
https://www.copper.org/publications/newsletters/innovations/1999/07/shape.html
https://en.wikipedia.org/wiki/Shape-memory_alloy
http://www.engineeringexpert.net/Engineering-Expert-Witness-Blog/tag/metal-atoms
Written by: Finn Amend