Two connected balloons inflated to different sizes behave contrary to many students’ expectations. Air from the smaller balloon goes into the larger one, increasing its size. This happens because the surface tension for the smaller balloon is greater than the surface tension of the larger one.
Materials:
- Two Balloons
- Surface tension apparatus (metal piping with two rotary valves and two nozzles to attach balloons)
- Hand air pump and tube (connected to apparatus)
- Tape to securely attach the balloons and prevent air leaks (duct tape works well)
- Stand to hold it up
Demo:
- Start with the valve open and both balloons deflated.
- Add air until both balloons are almost perfectly spherical.
- Close the valve connecting balloons and continue adding air to one of the balloons until its size becomes 3-4 times larger than the smaller one.
- Open the connecting valve. The smaller balloon will contract, forcing air inside the larger one.
Explanation:
If a balloon is mostly spherical, we can estimate the behavior of the pressure inside as its radius grows with the formula:
where
= pressure inside a balloon with respect to its radius (
)
= initial pressure in balloon (
)
= non-dimensional constant unique to the balloon (i.e. a number used to match experimental data with the formula)
= radius of balloon before inflation (
)
= final radius of balloon (
)
This formula shows that the pressure inside increases sharply when the radius of the balloon is close to its original size (), but as the radius grows and passes a certain value, the pressure will decrease, and continue to do so for all values of
, as shown in Figure 2. It should be noted that the formula is only accurate for radii within
.
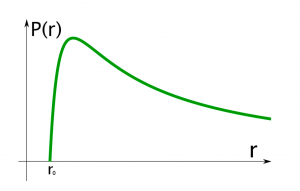
Fig. 2 Pressure curve for an ideal rubber balloon, from Masa Sakano.
You have probably experienced this phenomenon yourself when blowing up a balloon. When you first start to blow, it can be extremely hard to inflate. This is because you have a small radius and the pressure increases rapidly. When the balloon is small the pressure is more concentrated over the surface area. Once you force more air in the balloon it passes a threshold and becomes easier. When it is larger the balloon has a greater surface area and thus has less pressure.
For more information on the derivation of the formula above, see The Pressure Curve for a Rubber Balloon by Dr. Merritt and F. Weinhaus.
Notes:
- Make sure to test this demo before you perform it in class.
- Sometimes the elasticity of the balloons are different, this makes it difficult to inflate both at first.
- Check for leaks.
Written by Nick McCabe and Aleksander Beck
